Spiral membranes
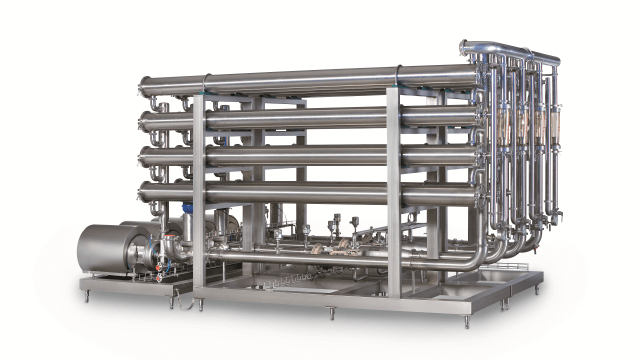
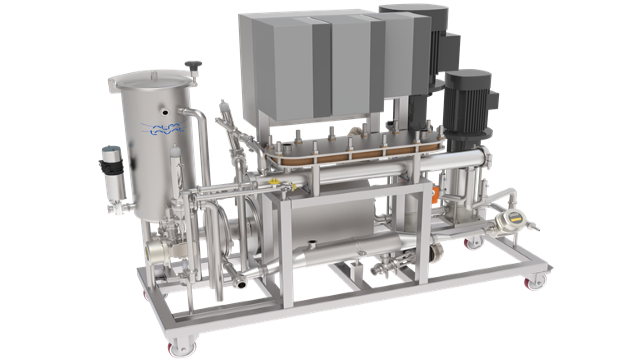
A wide range of full-fit, sanitary spiral membranes for reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF) and microfiltration (MF) to efficiently recover, purify, fractionate or concentrate products in industries such as food, beverage, dairy, biotech/biobased chemicals and pharma. They can also be used in water recovery. Different combinations of membrane, diameter, length and spacer ensure compatibility with all cross-flow membrane filtration processes.
Fine-tuned membrane filtration under the best possible flow conditions for efficient separation, superior flux and long service life
- Low initial investment and low replacement costs thanks to durable design
- Cost-effective – low energy consumption (compared to ceramic membrane) and compact design requiring minimal floor space
- Efficient – no need to replace/dispose of the cartridges or consumables used in traditional dead-end filtration
- Excellent chemical and thermal stability with high pH and temperature resistance
- Available as both flat sheet (for screening or lab work) and spiral (larger applications) to help you scale up smoothly
Our spiral wound membrane materials are compliant with all major regulations
All membrane materials used for both flat sheet and spiral wound configurations comply with EU Regulation (EC) 1935/2004, EU Regulation 10/2011, EU Regulation (EC) 2023/2006 and FDA regulations (CFR) Title 21, and are suitable for use in food and pharmaceutical processing applications. Compliance also extends to the related equipment and fittings, including items such as plate-and-frame units, element housings and pumps.

How it works - spiral membrane technology
The basic technology behind membrane filtration involves using a semi-permeable membrane to separate a liquid into two distinct streams.
Pumping this liquid across the surface of the membrane creates a positive trans-membrane pressure that allows any components smaller than the porosity of the membrane to pass through, forming the permeate.
Any components larger than the pore size simply cannot pass through, and remain behind in what is called the retentate. The surface of the membrane is kept free of blockages by the force of the liquid flow moving parallel to the membrane surface.
Efficient spiral membrane filtration
A spiral membrane comprises of a number of membrane 'envelopes' with 2 membrane sheets separated by a permeate spacer mesh, each glued to a central permeate collection tube.

Between each pair of envelopes there is a spacer which creates the feed channel, allowing the feed to flow across the length of the spiral membrane, whilst the permeate passing through the membrane into the membrane envelope flows in a spiral pattern to the permeate collection tube.
Red = Feed/retentate.
Yellow = Permeate.
Cost-effective – compared to a ceramic membrane
One of the key benefits of choosing a spiral membrane is that it is an economical solution compared to a ceramic membrane. It has both low capital and operating costs and at the same time the spiral membrane is ideal for high flow applications.